Molar Mass of Air
The atomic molecular weights are. What is the formula to find mass.

Ncert Exemplar Solutions For Class 9 Science Chapter 3 Atoms And Molecules Molecules Molar Mass Chapter
In practical calculations we may use the rough value of 00290 kgmol.

. Gm 3Dry air mostly consists of nitrogen 78 and oxygen 21 The remaining 1 contains many different gases among others argon carbon dioxide neon or heliumHowever the air will cease to be dry air when water vapor appears. The molar mass of water vapor is Mh00180153 kgmol and the rough value used in practical calculations is 00180 kgmol. Is the Boltzmann constant 1380 649 10 23 in JK 1 is the molecular mass of dry air approximately 481 10 26 in kg the specific gas.
Composition and content of each gas in air is given in the figures and the table below. What is the molar mass of dry air. The molar mass of dry air is dependent on the consistency of air but for standard air it is Mi0028964 kgmol.
The two most dominant components in dry air are nitrogen N 2 and oxygen O 2. Molar mass is defined from the ideal gas law Equation Mendeleev-Clapeyron. The formula for molecular weight of air.
This is written when solving the chemical equations as 2898 g mol or as 29 g mol. Kelly Eckert The Molar Mass of Air 102516 Introduction Air is all around. One way to calculate mass.
159994 x 2 319988 gmol remember it is 02 Argon. In practical calculations we may use the rough value of 00290 kgmol. The atomic molecular weights are.
The density of air is usually denoted by the Greek letter rho or ρ and it measures the mass of air per unit volume eg. The molar mass of dry air with oxygen nitrogen and the other components as indicated below is 289647 gmol. How do you calculate the mass of air.
If the formula used in calculating molar mass is the molecular formula the formula. The Chemical Composition of Air molar mass of dry air 00289652 kgmol Temperature at altitude h displaystyle h meters above sea level is approximated by the following formula only valid inside the troposphere no more than 18 km above Earths surubac and lower away from Equator. The average molar mass of air is 2898 grams per mole.
In chemistry the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula then adding all of these products together. The molar mass of dry air with oxygen nitrogen and the other components as indicated below is 289647 gmol. 140067 x 2 280134 gmol remember it is N 2 Oxygen.
The molar mass of water vapor is Mh 00180153 kgmol and the rough value used in practical calculations is 00180 kgmol. Therefore they are usually ignored when calculating the molar mass of dry air Gill P 2018. Oxygen and Nitrogen are diatomic in air O 2 and N 2 the molar mass of these gases can be explained as follows.
Citation needed air density kgm 3 absolute pressure Pa absolute temperature K is the gas constant 8314 462 618 153 24 in JK 1 mol 1 is the molar mass of dry air approximately 0028 9652 in kgmol 1. The mass of one carbon atom is equal to the atomic mass unit formerly abbreviated as amu but now spelled as u unified mass according to the latest IUPAC recommendation. To take into account the effect that these molecules have on air pressure you can calculate the mass of air as the sum of nitrogens two atoms of 14 atomic units each oxygens two atoms of 16 atomic units each and argons single atom of 18 atomic units.
The molar mass of dry air is dependent on the consistency of air but for standard air it is Mi 0028964 kgmol. Benzene - Thermophysical properties - Chemical physical and thermal properties of benzene also called benzol. However these elements are too small in volume to make significant molar mass changes in air.
For simplicity calculations for educational purposes this number is usually rounded to 29 grams per mole. View Lab Report - Molar Mass of Air from CHEM 130 at University of Kansas. Molar mass is a physical property defined as the mass of a given substance divided by its amount of substance so it is a weight of one mole of substance.
We know the percentage composition so we can use that to work out the weight of each component. What is the molar mass of dry air. Air is a mixture of several gases.
Calculate the molar mass of air. Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol. Calculate the number of moles of air in the atmosphere.
Air - Molecular Weight and Composition - Dry air is a mixture of gases where the average molecular weight or molar mass can be calculated by adding the weight of each component.

Molar Mass Definition Formula Mole Atomic Mass Chemistrygod

1 3 Calculating Molar Mass Of A Gas Using Pv Nrt Youtube

What Is The Relative Atomic Mass And Relative Molecular Mass Of An Element A Plus Topper Https Www Aplusto Relative Atomic Mass Molecular Mass Molecular

Molar Mass Definition Formula Mole Atomic Mass Chemistrygod
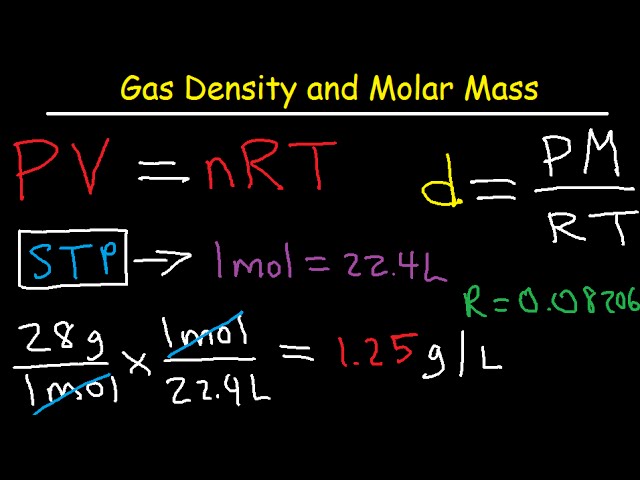
Gas Density And Molar Mass Formula Examples And Practice Problems Youtube
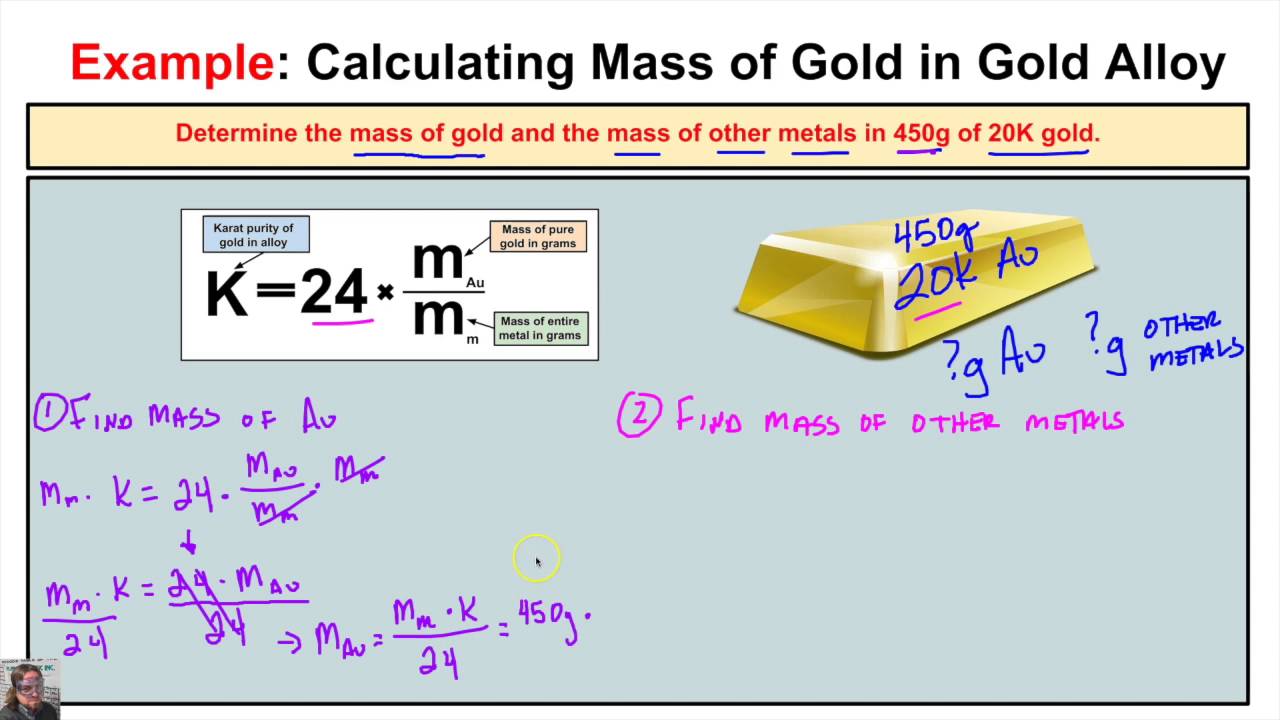
How To Calculate The Mass Of Gold And The Mass Of Other Metals In A Gold Chemistry Class Mass Chemistry
0 Response to "Molar Mass of Air"
Post a Comment